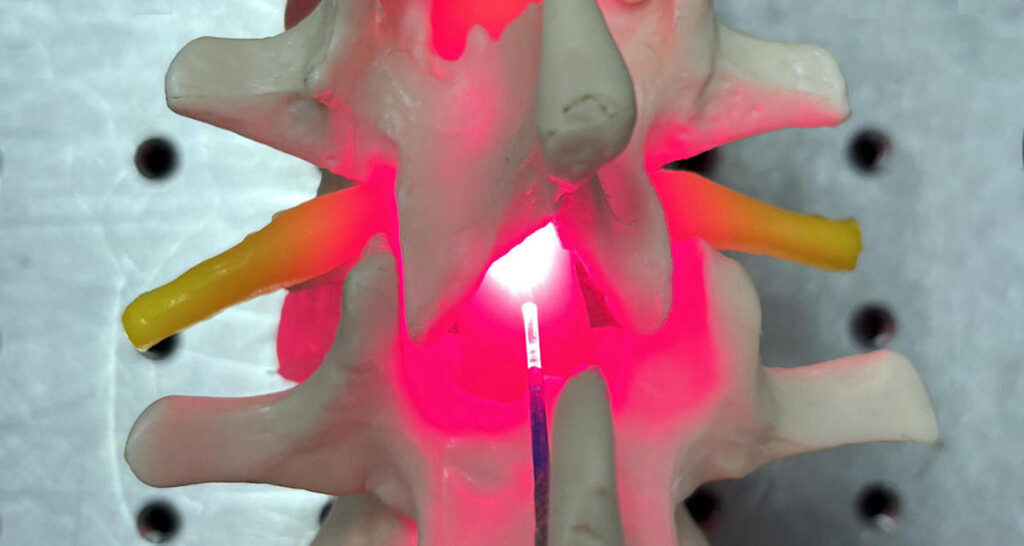
 Red Light Therapy –University of Birmingham
Red Light Therapy –University of BirminghamPeople with spinal cord injuries in the future could be healing their nerve connections with a device that uses red light and near-infrared light pointed at the exact source of damage.
The novel therapeutic approach, invented by scientists at the University of Birmingham, involves delivering light directly to the site of the injury through an implant.
Because surgery after spinal cord injury is very common already, doctors would have the opportunity, during the same operation, to implant the device that could treat and repair the spinal cord itself.
In the study, researchers determined an optimal ‘dose’ of light and showed that their method could deliver significant therapeutic improvements including significant restoration of sensation and movement, and regeneration of damaged nerve cells.
In just five days of treatment, they found that the delivery of red light at a wavelength of 660nm for one minute a day increased cell viability (a measurement of the number of live cells) by 45%.
“Excitingly, this aspect of the study showed the effect of 660nm light was both neuro-protective, meaning it improved survival of nerve cells, and neuro-regenerative, meaning it stimulated nerve cell growth,” said Professor Zubair Ahmed, who led the study.
BREAKTHROUGH: Yale Successfully Repairs Injured Spinal Cords Using Patients’ Own Stem Cells
Scientists used cell models of spinal cord injuries in adult rats to determine the frequency and duration of light required to achieve maximum restoration of function and to stimulate nerve cell regrowth, according to the results published in the journal Bioengineering and Translational Medicine.
The researchers also investigated the effect of light therapy in preclinical models of such injuries. Here they used two different methods, an implantable device and transcutaneous delivery, where the light source is placed against the skin. Their study showed comparable results for both delivery methods, with a one-minute dose of 660nm light, delivered daily for seven days, resulting in reduced tissue scarring at the site of injury, and significant functional recovery.
They also found significant reductions in both cavities and scarring as well as increases in the levels of proteins associated with nerve cell regeneration and improvements in the connections between cells in the injured area of the spine.
This is the first time transcutaneous and direct delivery of light have been compared in spinal cord injuries, and the results are a milestone for the researchers, who said there were “currently no approaches that preserve cells or improve neurological function”.
WORLD FIRST: Paralyzed Man Walks Again Using Device that Connects His Thoughts to His Spinal Cord
“Surgery after spinal cord injury is common, but currently these operations are only aimed at stabilizing injuries to the bones of the spine that have been damaged by the trauma,” said Neurosurgery Registrar Andrew Stevens, the first author of the study.
“This concept is incredibly exciting as it could offer surgeons the opportunity during the same operation to implant a device which could help protect and repair the spinal cord itself.”
Light therapy, called photobiomodulation (PBM), is backed up by evidence showing its effectiveness in a wide variety of dermatological and oral applications, where metered light dosing can be achieved with precision through direct-to-tissue delivery. For example, PBM is already NICE-approved for oral mucositis, where it has been shown to reduce the debilitating ulcers and painful inflammation in the mouth caused by cancer treatments.
‘CURRENT’ CURE:Movement in Paralyzed Arms is Restored by ‘Zapping’ Spinal Cords With Electrical Stimulation
Professor Ahmed explained that an implantable device is required to provide both line of sight to damaged tissue and the opportunity for greater accuracy without impedance due to the thickness of skin and tissues surrounding the spinal cord.
Since acquiring a patent through the University of Birmingham Enterprise, the researchers have already received further funding to develop an implantable device for human clinical trials on patients with traumatic spinal cord injury.
WALK THIS GOOD NEWS Over to Social Media – Share It…
Source link

